Special report: octaplasLG®
Around-the-clock production of
virus-inactivated pooled plasma
Next

The manufacture of octaplasLG®, our virus inactivated and prion-reduced pooled plasma, is a 24-hour process; production has to be done within a day to preserve the plasma proteins.
I joined Octapharma in 2012 as a process operator, later becoming first operator on the night shift, which developed into my current role as process specialist. My main responsibilities are to make sure that the processes are running, that the equipment is working and, ultimately, that the product is made.

Each frozen bag of octaplasLG® has a unique identity and is scanned individually during final packaging.
The most important steps in the production of octaplasLG® are the pooling and the virus inactivation. We pool 600–1,500 individual plasma donations in an 800-litre stainless steel vessel. From that, we produce an average of 3,600 bags of octaplasLG®, which is 780kg of plasma. Pooling plasma gives us a standardised product. Pooling levels out individual heterogeneity of single plasma donations and reduces the variability of plasma components, resulting in consistent product quality.
Here in Stockholm we produce octaplasLG®, which is named because of the use of affinity ligand gel (LG) chromatography in the prionreduction step. There are 27 of us working on octaplasLG® production in Stockholm and we operate in three shifts – day, afternoon and night. Everyone who works on octaplasLG® production has an impact on producing that batch. We currently run four batches a week, which is planned to increase to five.
Working on the production of octaplasLG®, we get to see the whole process from start to finish. Our process involves the pooling of individual donations; filtration; virus inactivation; purification; prion reduction; sterile filtration and aseptic filling. Our job is to preserve the proteins: to do so, we regulate the temperature and pH (acidity level). Throughout the process, the ingredients are stirred continually to ensure a homogenous product. We do everything, from thawing the plasma, right through to the point when it is frozen and packaged as a final product, ready for patients.
24 hours
to manufacture600–1,500
single donations are pooled60 minutes
of viral inactivation3,600 bags
of plasma in a batch
Discussing a chromatography column with a colleague.
OctaplasLG® is used during major operations and for major blood loss. There are also long-term patients who need regular plasma exchange. Without our plasma donors, we don’t have any product, so the act of donation is one of the most important contributions for patients. Knowing that the raw material we use is from plasma donors makes me feel more motivated to take care of it, and find new ways of improving production efficiency.
We recently underwent a major change by switching from one large chromatography column to small, parallel columns. This is more efficient both for maintaining the columns and for the process operators. There was a lot of planning leading up to the scheduled annual summer shutdown. I helped out with the installation, running the tests, and assisting in the cleaning validation. The validation batches, the first three batches we made after the change, demonstrate that the new process fulfils the requirements and product specifications.
I like the variety of my job most. One day I can be assisting with packaging, the next day I might be packing a column and on another day I may be abroad, doing a factory acceptance test (FAT) for a new machine. It’s a great mix between administrative and practical assignments. I enjoy finding new ways to improve efficiency using the principles of lean production. In my spare time, I am studying for a degree in technical engineering.
The manufacture of octaplasLG®
Pooling 600–1,500 individual plasma donations in an 800-litre stainless steel vessel
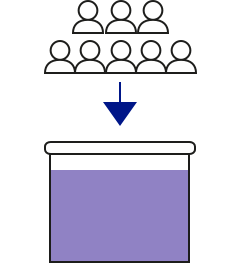
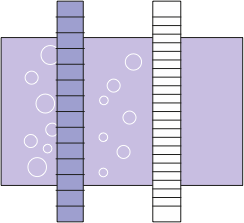
Filtration removing cells, fragments and aggregates
60 minutes of virus inactivation via solvent detergent (SD) technology
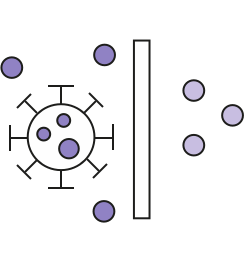
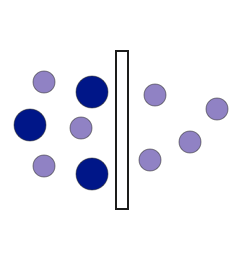
Purification chromatography step is used to remove solvent detergent components: SD removal step
Prion reduction Affinity ligand gel (LG) chromatography
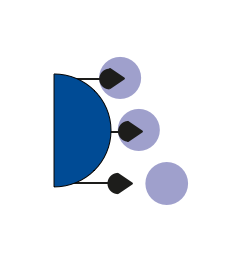
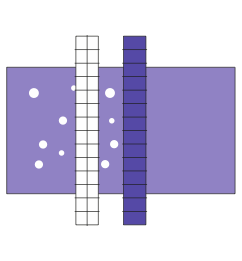
Sterile filtration to produce a sterile pharmaceutical product for patients
Aseptic filling of octaplasLG® into bags

Labelling, vacuum packaging and freezing
Testing & Release

Control of temperature, pH and coagulation factor content throughout process
